Research
Viruses are small infectious agents that require a host to replicate (obligate intracellular parasite). Viruses infect all living things but are not considered living organisms. They are incapable of producing energy and lack ribosomes, molecular machines required to build proteins.
The sole purpose of a virus is to infect a susceptible cell, reproduce, infect more cells, and spread to other hosts. In this manner, viruses are capable of environmental adaptation and evolution. They consist of genetic material protected by a protein coat (capsid).
Viruses possess either DNA or RNA genomes and some acquire a layer of fat (envelope) as they exit an infected cell. Many human viruses that cause disease are enveloped RNA viruses: Dengue, Ebola, HIV, Influenza, Measles, SARS-CoV-2, and Zika.
On this page:
HIV-1 Infection and AIDS
Human Immunodeficiency Virus (HIV) remains a global concern. Greater than 39 million individuals around the world are currently living with HIV (WHO). HIV infects and destroys CD4+ T cells, leading to the development of Acquired Immunodeficiency Syndrome (AIDS). Two major types of HIV exist: HIV-1 and HIV-2. HIV-1 causes the more severe form of disease and is the primary cause of the worldwide HIV/AIDS epidemic. HIV-2 is less pathogenic and is largely restricted to West Africa and Southern Asia. Both HIV-1 and HIV-2 emerged from separate cross-species transmission events from primates into humans and can spread from person to person by sexual contact, blood exchange, or via mother to child (vertical transmission). Untreated HIV has three distinct phases: an acute phase (flu-like symptoms; several days to weeks), a variable asymptomatic phase (several years) and a symptomatic/end-stage phase (AIDS). Progression to AIDS leads to impaired immune function, with patients usually succumbing to opportunistic infections or cancer. No vaccine for HIV exists. Current treatment for HIV consists of oral antiretroviral (ART) therapy, taken daily. ART includes a cocktail of three drugs that inhibit discrete steps in the viral replication cycle. ART typically reduces HIV to undetectable levels, though this treatment is not a cure. ART can lead to side effects and patients much take medication for the rest of their lives. New HIV drugs are still being developed, including those that are injectable. Most HIV infections occur in sub-Saharan Africa. However, thousands of new infections still occur every year in the United States. African Americans, Hispanics, Men who have sex with Men (MSM), and people living in the Southeastern United States are disproportionately infected with HIV-1.
Retroviruses
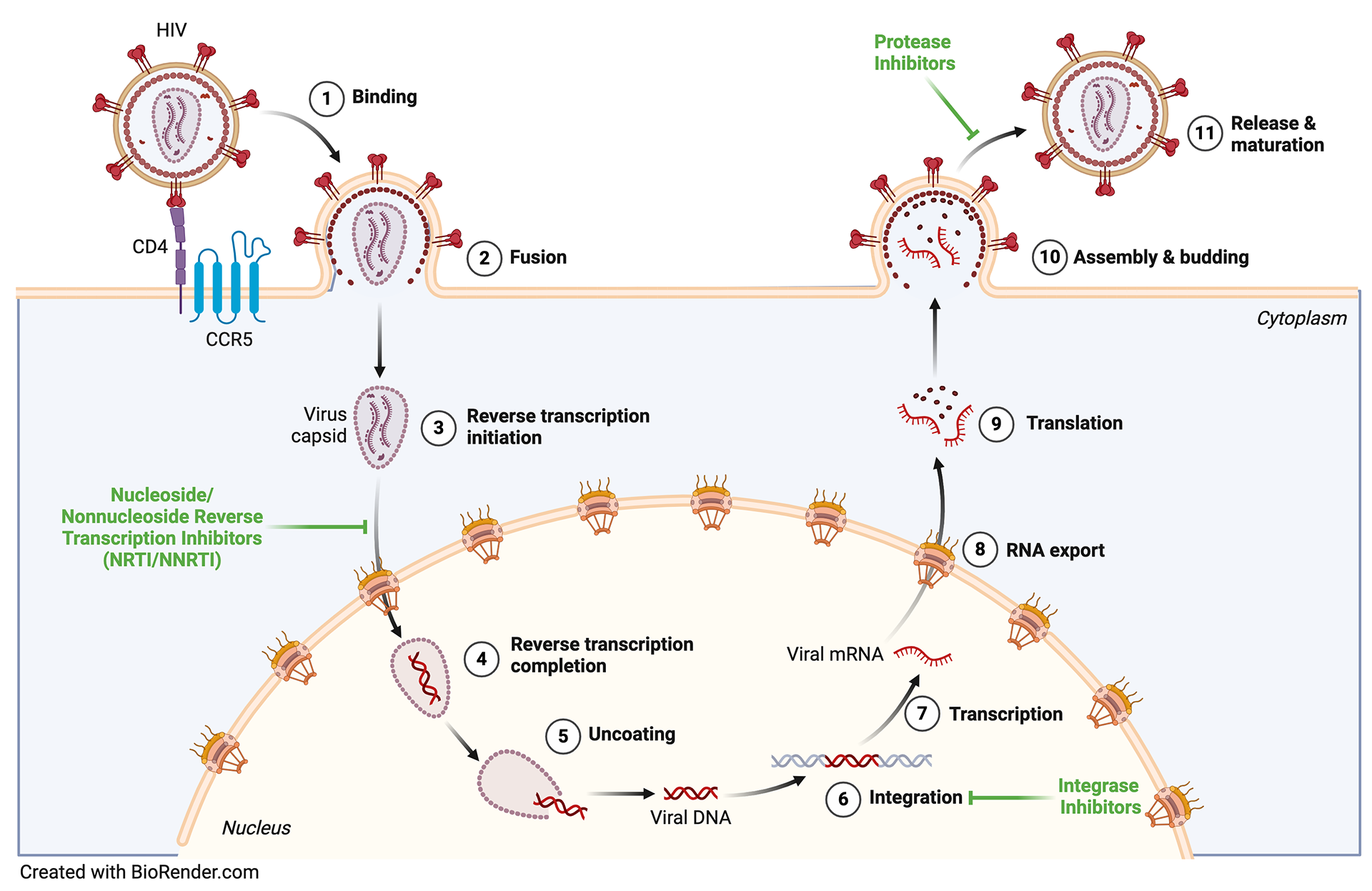
HIV is part of the Retroviridae family. Retroviruses received their name based on the unusual way these viruses replicate. All living organisms follow the central dogma of molecular biology, a theory that states genetic information flows in one direction, from DNA to RNA to protein. Upon infecting a cell, retroviruses use a virally encoded enzyme, reverse transcriptase, to convert their RNA genome back into DNA, thus backward (or retro) from the usual flow of information. Retroviruses with simple genomes (Avian Leukemia Virus; ALV) encode viral genes to build a virus particle (gag), facilitate viral replication (pol) and spread to new host cells (env). Complex retroviruses (HIV) encode additional accessory genes that aid in viral gene expression and modulating the host immune response. Retroviruses are thought to be over a half billion years old. Interestingly, some ancient retroviruses possessed the ability to infect our ancestors' germ cells (sperm and egg), leading to their endogenization (fossilization). Today, endogenous retroviruses (ERVs) make up about 8% of the human genome and play diverse roles in human health and disease.
Current Research Projects
Defining host factors required for HIV-1 Nef pathogenesis
HIV-1 Nef is a non-structural accessory gene necessary for viral disease (pathogenesis) and progression to acquired immunodeficiency syndrome (AIDS). Nef localizes to membrane-associated compartments, performing many well-conserved pro-viral functions including downregulation of cell surface proteins such as CD4 and MHC class 1 (MHC-I), modulation of T cell activation and autophagy, secretion into the extracellular environment via exosomes, and enhancement of viral infectivity and replication. Expression of Nef leads to a more infectious virus particle (virion). Thus, Nef modifies the viral producer (i.e., infected) cell so that virions are maximally infectious. Rhesus macaques infected with nef-deficient Simian Immunodeficiency Virus (SIV) display greatly reduced viral loads. Importantly, Nef alleles taken from HIV-1+ individuals at different stages of disease progression maintain the ability to enhance infectivity and viral replication. This implies that understanding the basis of Nef enhanced infectivity and viral replication remains an important medically relevant problem. We identified a host protein that Nef may require to enhance viral infectivity and replication. We are currently determining how Nef recruits this "dependency factor" to sites of viral assembly, the molecular determinants required for this interaction, and the effects of this interaction in cell lines and primary T cells.
Targeting long noncoding RNAs (lncRNAs) for HIV-1 latency reversal
The existence of dormant (i.e., latent) viral reservoirs within long-lived CD4+ T cells represents the major barrier towards a human immunodeficiency virus type 1 (HIV-1) cure. Reactivating and then eliminating HIV-1 remains a popular strategy. This "shock and kill" approach relies on identifying latency reversing agents (LRAs) that are strong enough to induce reactivation but will not lead to systemic immune activation. Long non-coding RNAs (lncRNAs) serve as attractive targets for HIV-1 latency reversal because of their cell, tissue, and disease specific expression. LncRNAs are RNAs greater than 200 nucleotides long that are not translated into protein. They possess many biological properties, acting like "molecular sponges" that bind ribonucleoprotein complexes to regulate gene transcription. Several lncRNAs have been implicated in HIV-1 pathogenesis. In a collaborative effort, we are conducting CRISPR/Cas9 screens to identify and validate lncRNAs that are dysregulated during HIV-1 latency and that interact with protein regulators of HIV gene expression.
SARS-CoV-2 Infection and COVID-19
Severe Acute Respiratory Syndrome (SARS-CoV-2) is the causative agent of Coronavirus Disease 2019 (COVID-19). SARS-CoV-2 emerged in late 2019, leading to a global pandemic that has infected over 775 million people and caused over 7 million deaths (WHO, 2024). Transmission of SARS-CoV-2 primarily occurs via respiratory droplets dispersed via infected individuals coughing, sneezing, and talking or by touching contaminated surfaces. Typical symptoms include fever, chills, and sore throat though a range of others can occur. Most individuals with COVID-19 will recover, though severe symptoms or death can occur in high-risk groups (unvaccinated, people over 60 yrs. old, individuals with high-blood pressure, diabetes, obesity, or those who are immunosuppressed due to HIV, cancer, or pregnancy). Several SARS-CoV-2 variants (Alpha, Beta, Gamma, Delta, Omicron) have emerged, with differing levels of contagiousness and virulence (disease). Vaccination provides strong protection against severe disease, hospitalization, and death. Various COVID-19 treatment options also exist to inhibit viral replication. Nonetheless, thousands of hospitalizations and deaths occur each week, including in the United States.
Coronaviruses
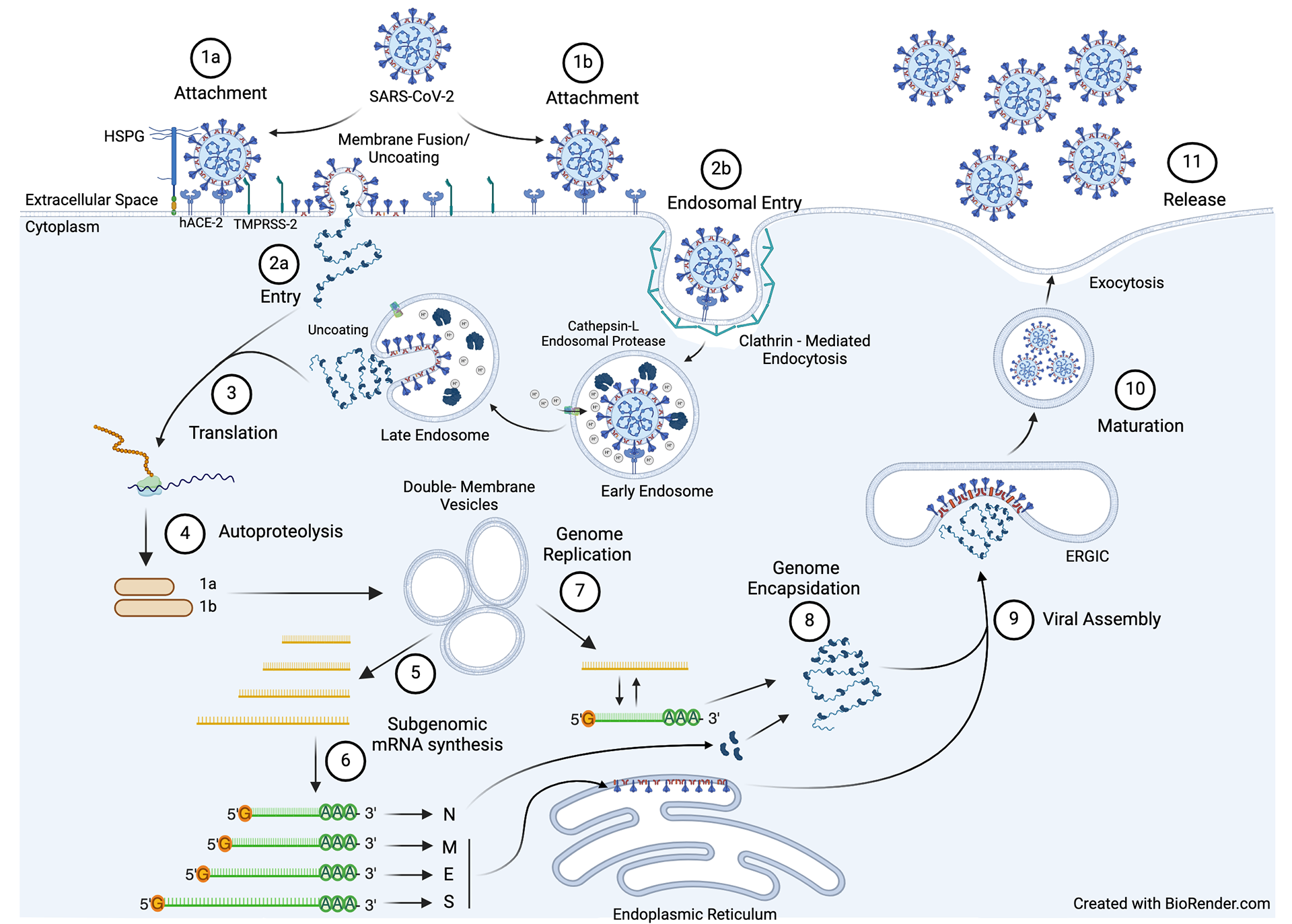
SARS-CoV-2 belongs to the Coronaviridae family. Coronaviruses are enveloped RNA viruses that infect a wide variety of mammals and birds. They received their name from club-shaped spikes emerging from their viral envelope, giving them the appearance of a solar corona when observed via electron microscopy. Coronaviruses possess the largest RNA genomes ever discovered. Seven human coronaviruses exist, leading to either mild (229E, NL63, OC43, HKU1) or severe (SARS-CoV-1, MERS, SARS-CoV-2) respiratory disease.
Current Research Projects
Delineating host factors required for SARS-CoV-2 replication
SARS-CoV-2 Spike mediates entry into mammalian cells and is the target of neutralizing antibodies induced via natural infection or most COVID-19 vaccines. Thus, identifying host factors that facilitate the intracellular trafficking of Spike is important for an increased understanding of coronavirus biology and for vaccine design. Specifically, we are studying: 1) the prevalence of the Spike/host CD4 interaction and its relevance to infection of T helper cells and 2) how a host protein may facilitate SARS-CoV-2 assembly/infectivity.
Using electromagnetic waves to inactivate pathogenic viruses
Standard pathogen disinfection includes the use of high temperatures, ultraviolet and ionizing radiation, and chemical agents. Electromagnetic waves offer an alternative strategy to inactivate viruses, possessing high penetration, uniform heating, and minimal pollution. Using electromagnetic waves therefore has precedence for sanitation and prevention efforts to reduce the transmission of pathogenic viruses. We are collaborating with a local company (Epirus) to determine if their technology, which uses electromagnetic waves, reduces the infectivity of SARS-CoV-2 Virus Like Particles (SC2-VLPs). We also aim to determine the mechanism for how this occurs. SC2-VLPs are non-infectious and encode a reporter transcript (luciferase) and all the SARS-CoV-2 structural proteins (Spike, Envelope, Matrix, and Nucleocapsid), recapitulating authentic aspects of SARS-CoV-2 entry, assembly, and release. We plan to extend our findings to other medically important enveloped viruses.
Testing sulfoglycodendrimers (SGDs) as broad-spectrum antivirals
Heparan sulfate proteoglycans (HSPGs) consist of cell surface proteins that possess unbranched negatively charged heparan sulfate (HS) polysaccharides. They are expressed in a wide variety of tissues/cells and possess many biological activities involved in angiogenesis, development, and cell homeostasis. Diverse viruses bind HS, including HIV-1 and SARS-CoV-2. This concentrates viruses at the cell surface, aiding attachment to cell receptors and entry into host cells. Sulfoglycodendrimers (SGDs) act as synthetic mimics of HSPG to inhibit viral attachment/entry. In collaboration with researchers at Sacramento State (Dr. Katherine McReynolds), we are determining whether select SGDs reduce the infectivity of SARS-CoV-2 Virus Like Particles (SC2-VLPs) and HIV-1 in both cell lines and primary cells. The goal would be to develop SGDs into a potential microbicide or spray, applied to mucus membranes to prevent virus transmission.